Safey & Efficacy Testing Services Understand the human responses to your compound
Available Models
Select from our standard models or customize your own.
2-Organ
Base efficacy & toxicity models

Heart-Liver
An effective test of the acute and chronic effects on the heart and liver. This is our foundational model.

Neuromuscular Juction (NMJ)
Better understand disorders impacting the connection between the nervous an muscular systems, such as ALS.
3-Organ
A more complete view of the human body

Heart-Liver-Cancer
Understand the effects of both single drug and drug-drug combinations to determine what best treats cancer while monitoring the side effects.

Heart-Liver-Skin
Evaluate the safety and efficacy of topically applied products.
4-Organ
Our most advanced, standard systems
Heart-Liver-Skeletal Muscle-Neuron
Investigate and get a complete toxicity and efficacy profile for your neuromuscular junction therapy.
Custom Models
Reconfigure the platform to include the relevant organ tissues for your application.
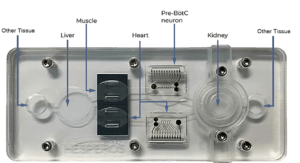
5-Organ+
The platform can be easily configured to include virtually any organ, barrier tissue, or tumor. Schedule a webinar with our research team to determine the model that will best fit.
Available Barrier Tissues
Incorporate any of the following barrier tissues to better understand how your compound is absorbed and secreted.

Blood-Brain Barrier (BBB)
Our BBB model, composed of iPSC-derived cells, has been extensively characterized for tight junction-mediated barrier formation and paracellular (passive) and transcellular (active) transport mechanisms. This module is well-suited for investigating the transport rates, transport mechanisms and barrier effects of novel chemicals and biologics.

Skin
Using Strat-M® membranes, a synthetic human skin module, integrated into our multi-organ systems, we can determine the transdermal diffusion of novel compounds applied topically and monitor the effects of the absorbed drugs on organ physiology in the system.

Kidney RPT
Using primary renal proximal tubule cells, the tubular reabsorption or secretion profile of a novel compound can be determined for excretion purposes. Additional data regarding the kidney safety profile of a drug can be collected in the form of barrier integrity changes (noninvasive TEER measurements) and release of the biomarker kidney injury marker-1 (KIM-1).

Gastrointestinal Tract
Using either iPSC-derived enterocytes, or immortalized patient biopsy intestinal epithelial cells (hiECs), the absorption and first-pass metabolism characteristics of a novel compound can be determined. Our hiEC model has been characterized for CYP activity and barrier formation.
Immune System
Monocytes / Macrophages can be added to the recirculating medium in our multi-organ systems. These cells (THP-1 monocytes) have been characterized in systems for expression of the lineage markers CCR5, CD14 and CD16 as well as markers of activation including CD11b, CD69 and CD86 following exposure to lipopolysaccharide (LPS) and interferon-gamma (IFN-γ). Additionally, following exposure to LPS and IFN-γ the monocytes have been shown to differentiation into macrophages and adhere to damaged tissue modules. Differential cytokine release profiles have also been characterized in the context inactivated, tissue damage-activated and cytokine storm activated conditions.
Included with Every Project
You can expect the following deliverables with every project.
Frequently Asked Questions
Explore
Get in Touch
Hesperos, Inc.
12501 Research Parway, Suite 100
Orlando, Florida 32826
(407) 900-5915
info@hesperosinc.com

